 NOTICIAS
NOTICIAS
Nuestro propósito es ayudar a prevenir infecciones y mejorar la calidad de vida de las personas
Presentes en más de 35 países
Más de 50 partners en todo el mundo
Productos I+D propia lanzados en 2021
Líderes en Clorhexidinas coloreadas
Conoce nuestros productos
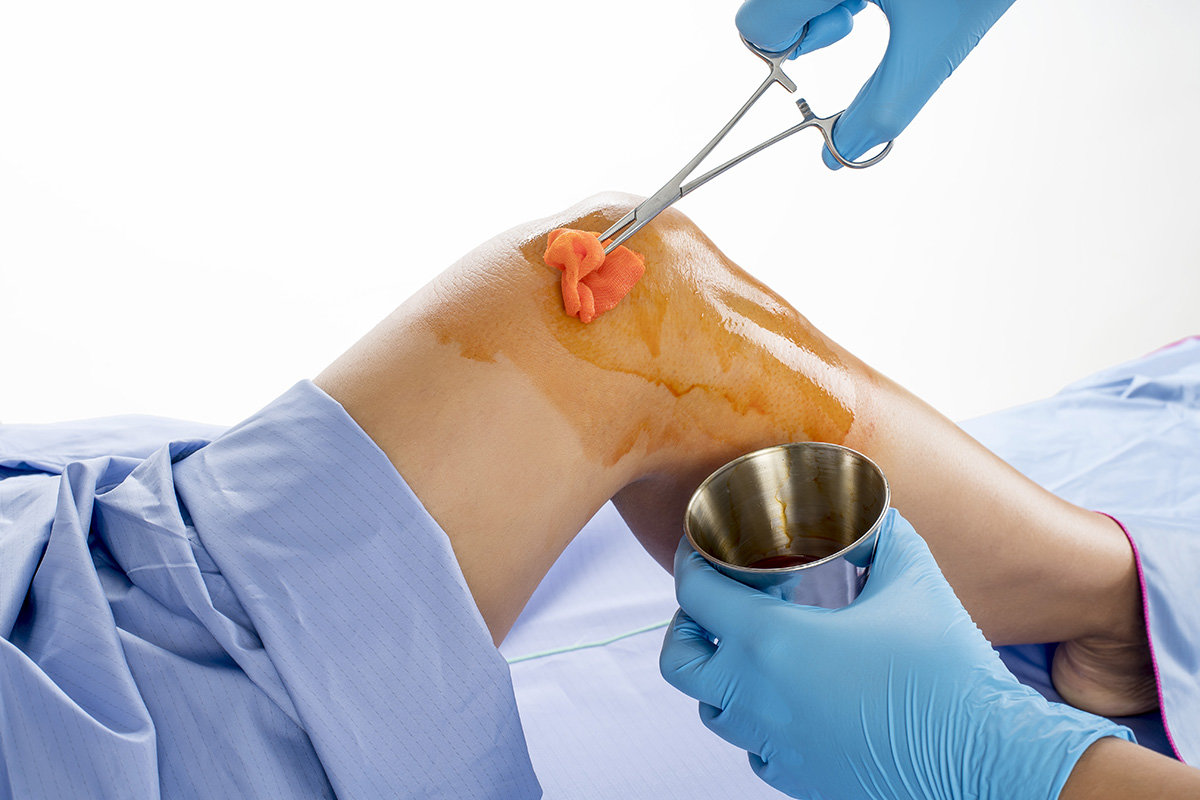
Disponemos de la más completa y variada gama de productos para la Prevención de Infecciones: antisépticos en base a clorhexidina, líderes en el mercado; desinfectantes para superficies; soluciones de desinfección de Alto Nivel para dispositivos médicos y equipamientos basados en las tecnologías más avanzadas.
Por gama
Por sector
Tu aliado en la lucha contra las IRAS
Acompañamos a los profesionales de Prevención y Control de Infecciones.
Ver másNovedades
 NOTICIAS
NOTICIAS
 NOTICIAS
NOTICIAS
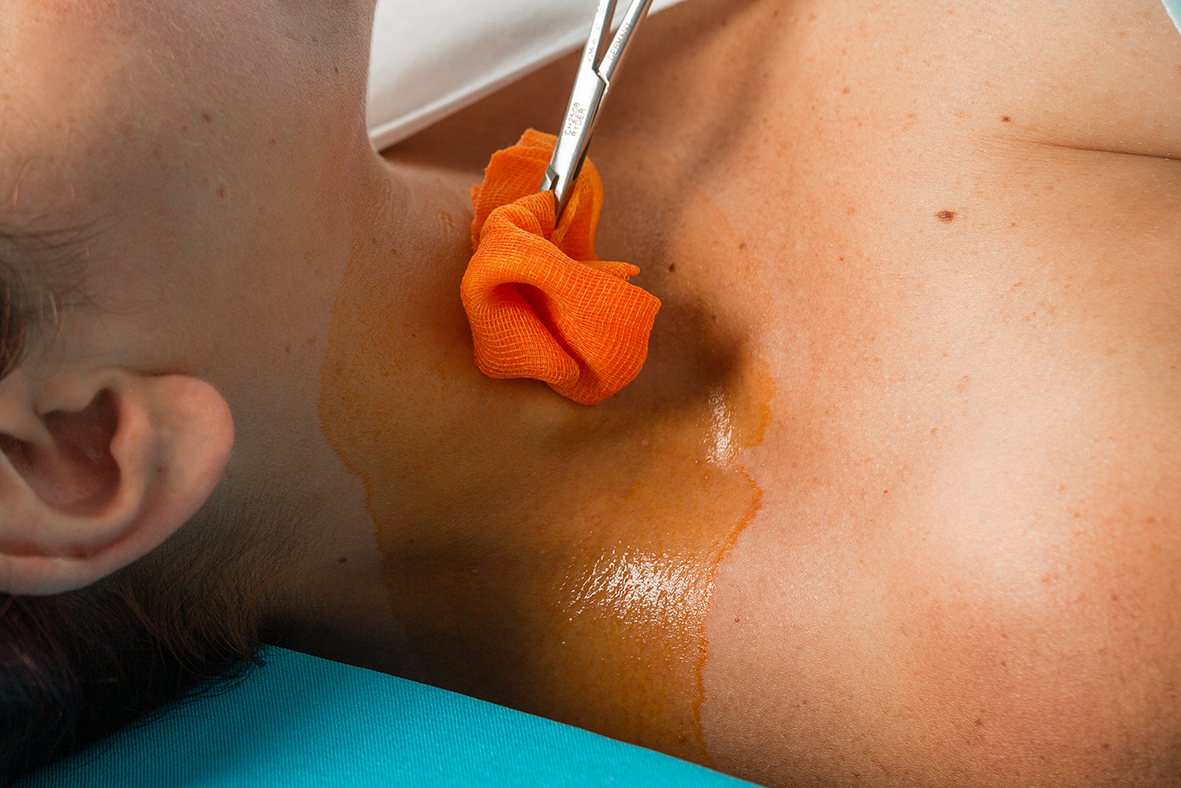
Antisépticos de uso prequirúrgico y punto de inyección: coexistencia de Medicamentos y Biocidas durante un período transitorio
Desde el 1 de junio de 2022, los antisépticos destinados al campo quirúrgico preoperatorio y a la desinfección del punto de inyección se consideran Medicamentos. ÚLTIMAS ENTRADAS
ÚLTIMAS ENTRADAS
Vesismin lanza en España el uso de la octenidina en el tratamiento de las heridas
Debido a que la piel está constantemente expuesta a posibles lesiones, la curación de heridas es un proceso fundamental para la supervivencia de todos los órganos. ÚLTIMAS ENTRADAS
ÚLTIMAS ENTRADAS
Desagües hospitalarios: un reservorio oculto de las IRAS
Los desagües se han identificado como importantes reservorios de patógenos, sobre todo de las bacterias gramnegativas multirresistentes.Unión Europea
Fondo Europeo de Desarrollo Regional
“Una manera de hacer Europa”
Vesismin SLU en el marco de Programa ICEX Next, ha contado con el apoyo de ICEX y con la cofinanciación del fondo europeo FEDER


 Higiene y Antisepsia
Higiene y Antisepsia
 Superficies
Superficies
 Instrumental
Instrumental
 Equipamiento
Equipamiento
 Accesorios
Accesorios